James Marsh
James Marsh (1794-1846)
James Marsh was an English chemist who developed a test for detecting the presence of arsenic in food, drink, and, more significantly, tissue; a test which, with a few alterations, remained in use for almost 150 years.
Professional life
Nothing is known about Marsh’s early life. He went to work at the Royal Laboratory at Woolwich Arsenal, probably at the age of twelve. When peace came in the 1830s, most of the young laborers were let go, but Marsh, because of his reputation and his contribution to the navy, was kept on.
He worked as a surgeryman and dispenser of medicines which meant he assisted the doctor. Despite the years working for them and the contributions he made, the Arsenal never made him a permanent member of staff. He was paid by the day, for a total annual salary of £82 13s. (By comparison, a professor, which Marsh occasionally did, was paid £200.)
During his career, Marsh was awarded the Royal Society of Arts’ Large Silver medal along with 40 guineas for his work on electromagnetism (in 1823), and later the Society’s Large Gold medal for his arsenic test. (He later became a member of the Royal Arts Society.) He was awarded a £30 bonus for inventing a crow’s quill percussion tube for the navy’s cannons; it increased their accuracy, and did away with the danger of the old copper percussion tubes (often expelled during the shot and wounding the gunners).
He worked as an assistant for Michael Faraday, professor of chemistry at the Royal Military Academy. Because of this association, Marsh became involved with the trial of John Bodle, accused of poisoning his grandfather, George. There was nothing remarkable about the case, and, if not for Marsh’s involvement, it would have fallen into obscurity.
The case that started it all
On Saturday, November 2, 1833 retired farmer George Bodle of Plumstead, Kent came down for his breakfast of coffee and toast. Shortly afterward he, and most of the household, became ill. Poison was suspected and Mr. John Butler, a surgeon from Woolwich, was sent for. George suffered from burning stomach pain, vomiting, and diarrhoea; symptoms shared by everyone who had drunk the morning coffee.
Though the rest of his household survived, three days after it began, eighty-one-year old George Bodle was dead.
Butler went to the local coroner, Charles Carttar to report his suspicions and ask for an inquest. Butler's suspicions were not the only ones that reached Carttar who called for an inquest. An autopsy was carried out by Mr Butler, Mr. Samuel Solly (lecturer of anatomy at St. Thomas’s Hospital), and Dr. Bossey, also of Woolwich. The autopsy was done in Mr. Bodle’s home.
As was the usual practice, evidence was sent to specialists for examination. In this instance, the contents of Bodle’s stomach and the remnants of the coffee grounds (suspected to be the mode of delivery) were sent to George Faraday at nearby Woolwich. Faraday considered the task too menial to fit into his busy schedule and passed it along to his assistant, James Marsh. Pressed for time, Marsh employed an old test of smell. He tossed some of the coffee grounds onto the fire and detected the smell of garlic. Other tests might have been performed (and likely were), but Marsh attested to being convinced of the presence of arsenic because of the smell, not because of any tests carried out.
The presence of arsenic in the nineteenth century did not prove poisoning (there was no shortage of accidental deaths or suicides by arsenic), and juries wanted to know how the poison was administered (a peculiarity that resulted in more than one acquittal when experts were unable to say for certain). In his testimony, all Marsh could say was that his tests demonstrated the presence of arsenic. It wasn’t enough for the jury, and John Bodle was acquitted.
Years later (1846 to be precise), when facing transportation for blackmail, John Bodle confessed to the murder.
In 1833, even without the confession, James Marsh was convinced George Bodle had been poisoned. But the tests at the time were ineffective and juries had certain expectations.
A sidestep should be made at this point. Arsenic is easily detected on its own. The difficulty facing chemists at the time was determining the presence of arsenic in tissue (that had already been absorbed by the body). Arsenic had to be separated from the biological matter first. In 1806, a Berlin pharmacologist named Valentin Rose had devised such a method. He boiled stomach tissue in distilled water to draw out any arsenic and then tested the solution for the poison. Unfortunately, awareness of his technique was limited.
Arsenic does not dissolve easily. If administered in solid form, particles would survive in the digestive tract. It was this evidence that juries required as proof of the presence of arsenic. But arsenic can be administered already dissolved in liquid, as it was in George Bodle case (dissolved in the morning coffee). This left no physical evidence in the digestive tract. There was no widely-known method to test for arsenic once it had been absorbed.
Not until 1836.
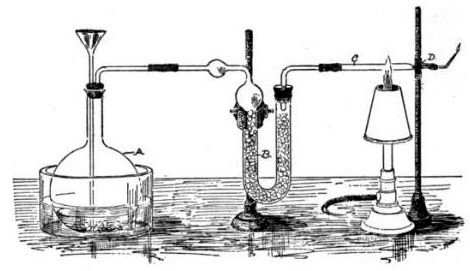
Marsh designed his test so that it would produce mirror arsenic which juries understood as definitive proof of the presence of arsenic. (When arsenic was heated in a test tube using a charcoal fire, dark shiny deposits of metallic arsenic formed on the cooler parts of the glass. This was referred to as mirrors.)
The test was sensitive enough to detect one grain of arsenic in four pints of water. (A grain is an old measurement for weight equivalent to 0.002 ounces or 64.798 mg; roughly it is the mass of a wheat or barley seed.)
Marsh published details of his test in the autumn of 1836 in the Edinburgh New Philosophical Journal.
Apparatus for the Marsh Test for arsenic.
Problems with the test
Despite it being a major victory in science, there were some difficulties.
The test, itself, was simple in theory, but difficult in execution. Marsh claimed that the test could be done by anyone in any household where zinc and sulphuric acid were available. In truth, it required no small measure of expertise to ensure the test was performed accurately. For example, If the glass was held too close to the flame, it would shatter. Further complications occurred with the material itself. Zinc and sulphuric acid were contaminated with arsenic which was used during the process of their manufacture. If the chemist did not ensure the purity of both, the test would provide a false positive.
Antimony, when put through Marsh’s test, appeared very similar to arsenic. The difference could only be distinguished through the colour of the mirror and that took a trained eye. This was significant because physicians would often administer a compound of antimony to the patient if they suspected poison. Antimony would induce vomiting which would expel the poison.
What was the impact of the Marsh Test
There are two parts to the answer; one for each area at play.
Within toxicology, the impact was substantial. The ability to detect arsenic within tissue allowed toxicologists to declare that it had been the cause of death; not simply that it had been consumed. It allowed them to prove the mode of death caused by arsenic—the absorption, rather than its presence in the digestive tract. Just as significant, for Mathiey Orfila (the father of toxicology) it allowed chemists/ toxicologists to use what he referred to as “pure” samples; tissue taken from the liver, spleen, kidneys, muscles, blood, and urine, rather than being limited to the contaminated stomach contents or preserved vomit.
For chemists and toxicologists, the Marsh Test was the silver bullet they needed in their “fight” against poisoning. It was to have banished it from the earth and every person who committed murder with arsenic would be caught and punished.
The reality was not as simple.
Within the courts the impact was less spectacular. For juries, detecting arsenic was only the first part of the problem. It did not indicate whether it had been taken by accident or for suicide. Nor did it prove who had done the administering. The 1840s saw a spike in the number of arsenic cases brought to trial. As before, proving that arsenic was the cause of death did not guarantee a conviction.
This is not to diminish Marsh’s accomplishment. As stated, it was a test that, with a few alterations, was used into the 1970s.
The Ending
James Marsh died unexpectedly on June 21, 1846, at the age of 52. Cause of death was listed at “inflammation of the liver,” more correctly hepatitis. Because he had never held the official post of ordnance chemist, he was not eligible for a pension, which meant, neither was his widow. The family of a man who had contributed so much to science and the navy was left destitute. (After many letters and petitions, a small sum of money was awarded.) A shameful end to a man who had contributed so much.
Bibliography
Hawksley, Lucinda, Bitten by Witch Fever: Wallpaper & Arsenic in the Victorian Home. (New York, NY: Thames & Hudson, 2016).
Hempel, Sandra. The Inheritor’s Powder: A Tale of Arsenic Murder. (New York, NY: W. W. Norton & Company, 2013).
Parascandola, John. King of Poisons: A History of Arsenic. (Dulles, VA: Potomac Books, 2012).
Stratmann, Linda. The Secret Poisoner: A Century of Murder. (New Haven, Connecticut: Yale University Press, 2016).
Wagner, E. J. The Science of Sherlock Holmes. (New York, NY: John Wiley & Sons Inc., 2006).
Whorton, James C. The Arsenic Century. (New York, NY: Oxford University Press, 2010).